Potassium permanganate (KMnO₄), also known as permanganate of potash, is a potent oxidising agent. Common potassium permanganate uses include water treatment, medical applications, and industrial oxidation. Due to its reactivity, it is handled with care in laboratories and industries. Its powerful properties make it essential in various chemical and pharmaceutical processes.
In this blog, we explore the applications of Potassium permanganate and measures to handle it safely. Dive in to learn more.
What is Potassium Permanganate?
Potassium permanganate, also known as Condy’s crystal, is an inorganic compound with the chemical formula KMnO4. It is a purplish-black crystalline salt, which dissolves in water as K+ and MnO− ions to give an intensely pink to purple solution. Potassium permanganate is a powerful oxidising agent that is important in chemical manufacturing. It is on the World Health Organization’s List of Essential Medicines; estimated Global production in the year 2000 was at 30,000.
Overview and Properties
Chemical Properties and Composition
- Molar Mass: 158.03 g/mol
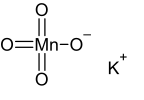
- Structural Formula: KMnO₄ Potassium permanganate consists of K⁺ and MnO₄⁻ ions. The MnO₄⁻ ion has a tetrahedral geometry.
- Hydrogen Bond Donor Count: 0
- Hydrogen Bond Acceptor Count: 4
- Reactivity: Strong oxidising agent. Reacts violently with organic materials, reducing agents, and acids. Decomposes upon heating, releasing oxygen gas.
- Functional Group: Permanganate (-MnO₄)
- Hybridisation: The manganese atom in MnO₄⁻ is in a sp³ hybridised state, forming a tetrahedral molecular shape.
Potassium Permanganate Structure
Potassium permanganate (KMnO₄) consists of a potassium ion (K⁺) and a permanganate ion (MnO₄⁻). The potassium permanganate structure consists of a central manganese atom bonded to four oxygen atoms in a tetrahedral arrangement.
Key Applications Of Potassium Permanganate In the Industry
Permanganate of Potash exhibits strong oxidising properties that do not generate toxic byproducts. Potassium permanganate uses include purification, medical treatments, and industrial oxidation processes. It is used in various industries as follows:
-
Water treatment
Potassium permanganate is broadly used in the water treatment industry. It is used as a regeneration chemical to remove iron and hydrogen sulfide, which results in a rotten egg smell from well water via a “manganese greensand” filter. It is commercially available as “Pot-Perm” at pool supply stores and is used to treat wastewater. KMnO4 is an attractive pre-oxidant for removing cyanobacteria because it is easily implemented and has historically been used in water treatment. In drinking water treatment, permanganate has been used to remove iron and manganese, control taste and odour-causing compounds, and for other applications like the control of zebra mussels attached to water intake pipes.
-
Synthesis of organic compounds
As a powerful oxidising agent, a significant application of KMnO4 is a reagent for the synthesis of organic compounds, resulting in the formation of carboxylic acids. Significantly used in synthesising ascorbic acid, chloramphenicol, saccharin, isonicotinic acid, and pyrazinoic acid. An alkaline potassium permanganate solution, referred to as Breyer’s reagent, is used in qualitative organic analysis to test for unsaturation in organic compounds. Another common use of potassium permanganate solution is in thin-layer chromatography (TLC) as a stainer for the detection of oxidisable functional groups, such as alcohols, aldehydes, alkenes, and ketones, which result in a white to orange spot on TLC plates.
-
Analytical reagent in the chemical industry
Potassium permanganate can be used to determine an aqueous sample’s total oxidisable organic material quantitatively. The value specified is known as the permanganate value. In analytical chemistry, a standardised aqueous solution of KMnO4 is sometimes used as an oxidising titrant for redox titrations. As potassium permanganate is titrated, the solution becomes a light shade of purple, which darkens as an excess titrant is added. Potassium permanganate was used as a bleaching agent in microscopic anatomy or microanatomy.
-
Fruit preservation

Ethylene absorbents lengthen the storage period of bananas when kept at high temperatures. Optimal storage of bananas happens when polyethene skin contains potassium permanganate during packing. The permanganate reaction with ethylene leads to its oxidation and blocks ripening processes, thus lengthening fruit storage by up to 4 weeks without cold storage requirements.
-
Pharmaceutical Use
Potassium permanganate treats various skin conditions, including fungal infections, impetigo, pemphigus, dermatitis, and topical ulcers, especially those with excessive fluid. It can be applied as a soaked dressing or bath and is safe for children and adults. It also helps clean radioactive contamination from the skin. Short-term use is advised to prevent irritation when treating eczema. To avoid nail discolouration, petroleum jelly can be applied before soaking.
Safety Measures For Handling Potassium Permanganate Crystals
Potassium permanganate poses risks as an oxidiser. Contact with skin can cause skin irritation and, in some cases, severe allergic reactions. It can also result in discolouration and clothing stains.
-
Handling Precautions
When handling potassium permanganate, always wear protective gear such as gloves, goggles, and a lab coat while ensuring proper ventilation to avoid inhaling dust or fumes. Direct contact with skin, eyes, and clothing should be avoided, and it must be kept away from combustible materials due to its strong oxidising properties. Direct mixing with organic substances, acids, or reducing agents can lead to hazardous reactions.
-
Storage Guidelines
Potassium permanganate should be kept in a cool, dry, and well-ventilated area in a tightly sealed, corrosion-resistant container, away from moisture, heat, and direct sunlight. It must be stored separately from flammable and incompatible materials such as acids and peroxides, with clear labelling and adherence to local hazardous substance regulations.
Where to Buy Potassium Permanganate?
Potassium permanganate is a potent oxidising agent widely used in pharmaceuticals, water treatment, and various industrial applications.
At Advent, we manufacture this chemical on a gram-to-kilogram scale. Our product is supplied to esteemed pharmaceutical customers domestically and internationally as a working standard, accompanied by complete characterisation data. The potassium permanganate price varies depending on purity, quantity, and supplier, with higher-grade versions used in pharmaceuticals and laboratories. For more information on fine and speciality chemicals, visit our website now.


