Ammonia and ammonium hydroxide are often used in fertilizers, for nutritional requirements for terrestrial organisms, for medications, and even in commercial cleaning goods. It may be difficult to foresee the long-term effects of these chemical components, yet they are quite popular all around the globe. Despite having lost their practical use, these chemicals are nevertheless suitable for our day-to-day activities.
However, there are so many distinctions between these two chemical substances that they must be handled separately. While the ingredients involved in each compound are different, they will always be made up of smaller parts. Even the purposes for which each chemical is used are distinct. Additionally, their formulas are different. You need to know the distinction between these two since it is by defining and distinguishing that you can learn about the difference.
Ammonium Hydroxide Manufacturers have been able to draw in and serve valuable customers with a wide selection of chemicals thanks to a group of chemical professionals that are dedicated to the work. The compounds listed here are carefully manufactured by using only the best basic chemical compounds and other needed materials, all of which are obtained from reputable suppliers in the industry.
Ensuring proper methodology is used, such as maintaining long shelf life, assuring accuracy in formulation, and monitoring and controlling pH value, enhances features like extended shelf life, reproducibility, and accuracy. Due to its aforementioned features, they have been able to serve the needs of paint, chemical, pharmaceutical, detergent, food, leather, and textile sectors.
Additionally, it is possible to determine the % ionization of the solution using the conductivity data. All of these approaches demonstrate that more neutral ammonia (and water) molecules are present in the solution than ammonium and hydroxide ions, thus these solutions are more correctly referred to as aqueous ammonia and not ammonium hydroxide.
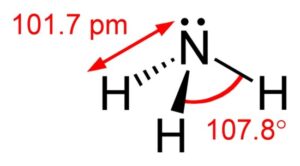
Ammonia is a weak base, whereas ammonium is a weak acid. The water molecule is a relatively mild acid while hydroxide is a very strong base. Water is present in ammonia hydroxide and that is why it is referred to as ammonia with water, ammonia liquor, and the like. Since ammonium hydroxide is a mixture of ammonia and water, this implies it is an ammonia solution. The quantity of ammonia in the solution is merely a little quantity, and the formula of the solution is NH3 (aq). Ammonium hydroxide is used in a wide variety of cleaning applications.
One example is that it is used as a component in window cleaners. It may be used as a component for cleaning products or it may be used as a cleaning agent alone. Because it is mostly employed as a cleaning agent, it is referred to as ‘ammonia’. The product is yellow and scented with the aroma of lemon, or it is green and scented with the aroma of pine. Ammonium hydroxide is commonly used in the meat business, particularly in the making of processed meats. It is a common practice to add a pH buffer that results in ammonium hydroxide, and also used by many industries is to supplement their meat process with a pH buffer that converts to ammonium hydroxide.
This is a partial list of some of the key distinctions between ammonia and ammonium hydroxide. And in addition, here is the definition and the formula for each. Having these resources provides you with a better understanding of the two composite elements. Many
Ammonium Hydroxide manufacturers provide a variety of Ammonium Hydroxide for industrial purposes, including rayon manufacturing, fertilizer production, refrigerant, and antifreeze manufacture, rubber manufacturing, medicines, soaps, lubricants, inks, explosives, and home cleansers. These items are prepared to utilize high-grade chemical components and state-of-the-art technologies.

