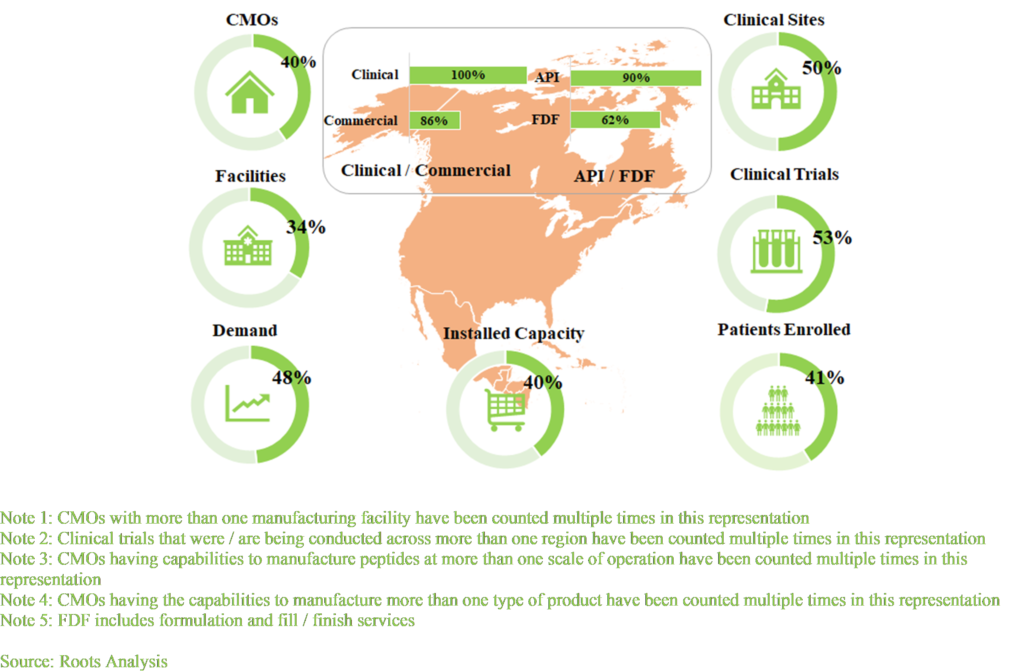
An informed capability assessment of different geographies, such as North America, Europe and Asia Pacific, is based on a variety of relevant parameters, such as number of CMOs, number of clinical sites, number of completed / active / planned clinical trials, number of patients enrolled, number of manufacturing facilities, number of CMOs offering manufacturing for API / FDF, number of CMOs having capabilities for clinical / commercial manufacturing, current demand for peptide therapeutics and installed manufacturing capacity in a particular region. Where, North America emerged as the most prominent region as per the above-mentioned parameters. It is worth highlighting that across all the regions, the peptide manufacturing facilities are capable of providing manufacturing services at the clinical scale.
The figure presents the analytical distribution of the above-mentioned parameters in North America.